Radiologia Brasileira - Publicação Científica Oficial do Colégio Brasileiro de Radiologia
AMB - Associação Médica Brasileira CNA - Comissão Nacional de Acreditação
 Vol. 43 nº 5 - Sep. / Oct. of 2010
Vol. 43 nº 5 - Sep. / Oct. of 2010
|
ORIGINAL ARTICLE
|
|
The value of diagnostic imaging in the classification of endoleaks as a complication of endoluminal grafting of aortic aneurysms |
|
|
Autho(rs): Francisco Abaeté das Chagas Neto1; André Rodrigues Façanha Barreto1; Henrique Ferreira dos Reis1; João Paulo Giacomini Bernardes1; Juliana Pinho da Costa Leitão2; Adson Freitas de Lucena3; Valdair Francisco Muglia4; Jorge Elias Junior5 |
|
|
Keywords: Aneurysm; Therapeutics; Complications; Diagnostic imaging. |
|
|
Abstract: INTRODUCTION
Aneurysm is the term utilized to describe a circumscribed dilation of a vessel or heart wall, that is larger than 50% of the presumed normal diameter(1). Among aortic aneurysms, 90 % to 95% are located in the abdominal aorta below the renal arteries bifurcation(2). The prevalence of abdominal aortic aneurysms increases with age, affecting approximately 6% of individuals after 65 years of age(3). The mean age at diagnosis is between 65 and 75 years, with male predominance(4). Currently, its incidence is increasing as a consequence of the global population aging(5). Studies indicate that its etiology is multifactorial (atherosclerotic, genetic, traumatic, infectious, inflammatory and degenerative) with the actual relevance of each one of these factors remaining controversial in the literature(6). In elderly patients, the conventional surgical treatment of abdominal aortic aneurysms presents a mortality rate ranging between 2% and 8%(7), being indicated in cases where: a) the abdominal aortic aneurysm is symptomatic; b) the aneurysm is larger than 5.5 cm, independently of symptoms; c) the aneurysm is larger than 6.0 cm in patients presenting with high surgical risk(8). In the early 90’s the experiments developed by Parodi et al.(9) originated the endovascular treatment utilizing the percutaneous placement of intraluminal prosthetic grafts, indicated for patients presenting with high surgical risk and favorable anatomy(8). The endovascular techniques utilized for the treatment of aortic aneurysms have become an excellent therapeutic option, with the future possibility of becoming the preferential approach for such disease as they are less invasive than conventional surgery, besides presenting satisfactory outcomes(10). The technological development of prosthetic grafts with fenestrated and branched systems has allowed the increase of indications in previously unfavorable situations(11). The purpose of the endovascular treatment is to achieve a complete exclusion of the aneurysmal sac by means of the placement of intraluminal prosthetic grafts. However, a frequent and feared complication is the persistence of blood flow in the aneurysmal sac after the endovascular repair (endoleak), observed in approximately 10% to 25% of cases, with spontaneous resolution in only 40% to 50% of these cases(12). The objective of the present study is to report a historical series of endoleak cases with emphasis on the diagnostic techniques (CT angiography and ultrasonography [US]), describing the current classification, and contributing to greater knowledge to promote an increase in the suspicion and diagnosis of this complication. MATERIALS AND METHODS A retrospective, descriptive study was developed with data collected in the data bank of the system of electronic reports and didactic files of a public university hospital in the São Paulo State, Brazil. Cases of endoleaks diagnosed by means of US and mainly by CT angiography at the institution in the period from 2005 to 2009 were selected. Twenty cases were selected and utilized to illustrate the different types of endoleaks. The studies were performed in a Somatom Emotion single detector row helical CT equipment (Siemens AG; Erlangen, Germany), adopting the following parameters: slice thickness: 3.0 mm; pitch: 0.5 and reconstruction of 1.0 mm; mAs: 100; kV: 110; CTDIw: 4.85 mGy; and with a Logic Pro 500 US unit (General Electric; Milwaukee, WI, USA) with a convex 3–5 MHz transducer. Non-ionic contrast medium (iodine concentration: 300 mg/ml) administered by means of an infusion pump through a peripheral venous access was utilized in the CT angiography, with a flow of 3–4 ml/s and total infused volume of approximately 150 ml, with a delay time of 25 seconds. The option was made to perform the post-processing of the axial images with multiplanar volumetric and three-dimensional reconstructions with multiplanar reconstruction (MPR), volume rendering technique (VRT) and maximum intensity projection (MIP) in workstations for a better documentation and classification of the endoleaks. The following parameters were investigated: gender, age, race, imaging method utilized for the diagnosis, affected region, aneurysmal sac dimensions and endoleak type, according to the modified White et al classification, consisting of: Type I – Blood flow to the endoleak originating at the coupling extremity of the prosthesis. It can be sub-classified as proximal (IA) and distal (IB). Type II – Blood flow originating from collateral vessels branching from the aorta filling the aneurysmal sac. Type III – Secondary to structural failure of the endoprosthesis. Fractures, orifices or modular devices separation. Type IV – Related to the stent porosity, being observed soon after placement of the prosthesis. Type V – Aneurysmal sac expansion without the identification of an endoleak. It is also known as endotension. The images were independently evaluated by at least two radiologists with the final consensus prevailing as the result. Subsequently, data were compiled and tabulated by means of a specific software and submitted to statistical and descriptive analysis. RESULTS Amongst the 20 patients diagnosed with endoleak, 14 (70%) were men and 6 (30%) were women. The race with greater prevalence was white (80%) followed by mixed (10%) and black (10%). As regards sample distribution by age, the mean value was 76.3 years ranging from 43 to 91 years. The most frequently involved vascular sites were the infrarenal abdominal aorta with 13 cases (65%), followed by the thoracic aorta with 4 cases (20%), the iliac territory with 2 cases (10%), and carotid vessels, with 1 case (5%). CT angiography was the imaging method preferentially utilized for the diagnosis and classification of the endoleaks in 17 cases (85% of the sample) distributed among types IA, IB, II and III; however US could detect IA type endoleaks in the other three cases (15% of the sample). The cross diameter of the aneurysmal sacs ranged from 3.8 to 9.8 cm (mean value of 5.8 ± 1.5 cm), the anteroposterior diameter ranged between 3.5 and 9.7 cm (mean value of 5.8 ± 1.4 cm), and the longitudinal length ranged from 4.6 to 20.0 cm (mean value of 9.8 ± 4.5 cm). Distribution of the samples according to endoleak types (Figures 1 and 2) 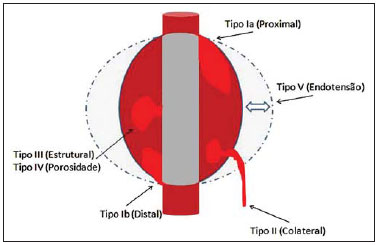 Figure 1. Scheme illustrating the different types of endoleaks, according to the classification proposed by White et al. 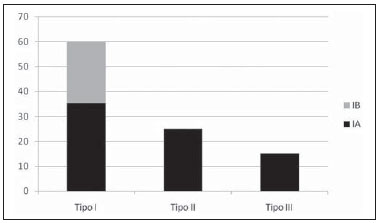 Figure 2. Sample percentage distribution chart as regards endoleak types classification. Type I endoleak presents with the blood flow originating at an anchoring point of the endoprosthesis. Sub-classifications were described as proximal (IA) or distal (IB) (Figures 3, 4 and 5). In the present sample,12 cases of type I endoleaks were observed, 7 of them being proximal (35% – type IA) and 5 distal (25% – type IB). 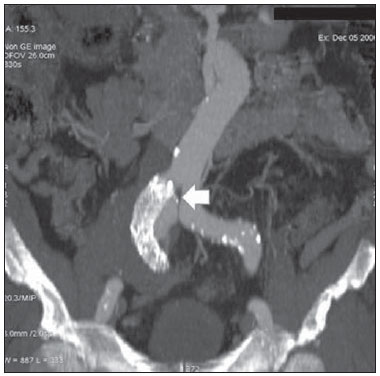 Figure 3. Volumetric reconstruction, in the coronal plane of abdominal aorta CT angiography demonstrating endoprosthesis in the aorto-bi-iliac segment and leakage of contrast into the aneurysmal sac in the proximal region of the prosthesis (endoleak type IA – arrow). 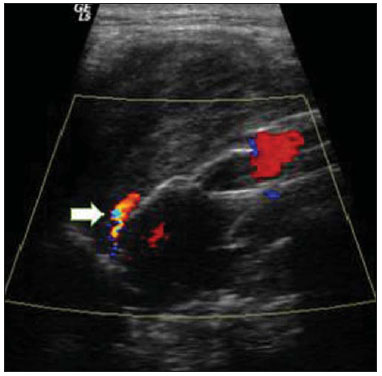 Figure 4. Color Doppler US of the abdominal aorta demonstrating endoprosthesis in the abdominal segment and peri-prosthesis flow in its proximal extremity (endoleak type IA – arrow). 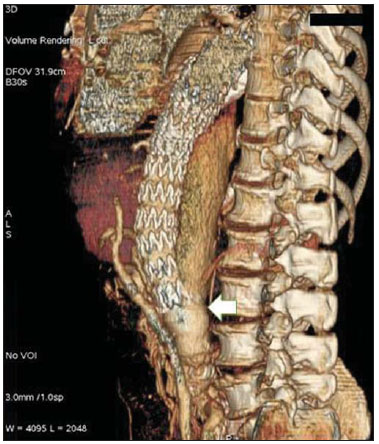 Figure 5. Three-dimensional reconstruction, in the sagittal plane of CT angiography of abdominal aorta demonstrating endoprosthesis in the abdominal segment and contrast leakage into the aneurysmal sac in the distal region of the prosthesis (endoleak type IB – arrow). Type II endoleak presents with the blood flow coming from collateral aortic vessels, filling the aneurysmal sac (Figures 6 and 7). Five such cases (25%) were observed in the present sample. 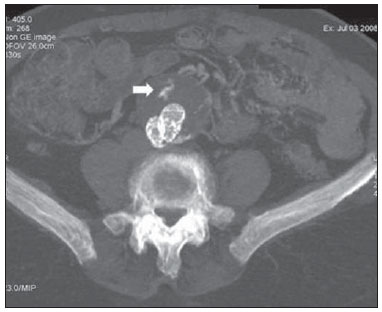 Figure 6. CT angiography of the abdominal aorta, axial section demonstrating endoprosthesis in good conditions and enhanced collateral vessel irrigating the aneurysmal sac (endoleak type II – arrow). 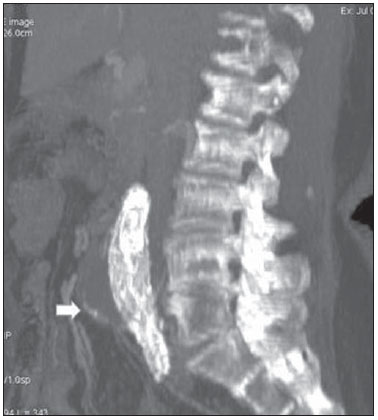 Figure 7. Volumetric reconstruction in the sagittal plane of abdominal aorta CT angiography demonstrating endoprosthesis in good conditions and enhanced collateral vessel irrigating the aneurysmal sac (endoleak type II – arrow). Type III endoleak is characterized by the structural failure of the endoprosthesis, whether such failure is due to partial or total detachment of its components in modular prosthesis or to fracture in such components causing a persistent flow into the aneurysmal sac (Figure 8). Three such cases (15%) were observed in this study. 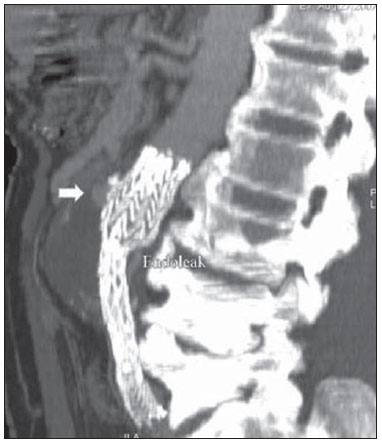 Figure 8. Volumetric reconstruction in the sagittal plane of abdominal aorta CT angiography evidencing structural failure of the endoprosthesis with leakage of contrast into the aneurysmal sac (endoleak type III – arrow). Type IV endoleak is related to the stent porosity, and can be observed soon after the placement of the prosthesis. It is also related to the anticoagulation status of the patient, and is generally spontaneously corrected, or after the coagulation time is restored to a normal level. The aneurysmal sac expansion without the identification of an endoleak is known as endotension or endoleak type V. Its cause is uncertain, being possibly related to another type of endoleak not detectable at the currently available imaging methods. Types IV and V endoleaks were not observed in the present sample. DISCUSSION The typical patient affected by this complication is the male elderly patient with infrarenal abdominal aortic disease(6). In the present series, type I endoleak was the most prevalent. However, in the literature, type II endoleak is reported as being the most frequently identified type(8). Endoleaks may develop during the procedure or sometime after it(13). The early diagnosis and type identification are extremely relevant, as in some cases endoleaks may be associated with the increased pressure in the aneurysmal sac and increased risk for rupture(14–17). Therefore, lifetime follow-up by means of periodic imaging studies is necessary to assure the success of the percutaneous treatment and the timely detection of complications representing peculiar conditions for the benefit of patients’ health(18). Currently, the classification most recommended by the vascular surgeon’s societies is the one developed by White et al., that was modified in recent years, dividing endoleaks into five types(19-21). This classification is based on the origin of the blood flow into the aneurysmal sac and has direct implication on the treatment required for each case. Most frequently, type I endoleaks occur after endovascular repair of thoracic aortic aneurysms. Additionally, they are most frequently found in patients with a complex arterial anatomy. Short necked, angled, ulcerated and trapezoid aneurysms containing mural thrombus, pose a challenge for the construction of appropriate seals between the prosthesis and the native aorta(22,23). Generally, the prognosis for type I endoleaks is reserved, and an aggressive treatment is mandatory. Surgical or endovascular interventions are recommended whenever a type I endoleak is documented between two and four weeks following endoprosthesis implantation. A type I endoleak located in the proximal neck region (type IA) is a very dangerous event, as the false lumen is continuously submitted to high pressures, increasing the risk for rupture. In such cases, an immediate intervention is mandatory, with the implantation of one or more prostheses(24). Type II endoleaks occur as the blood flows through the branches of the aorta segment that did not receive a stent or iliac arteries whose anastomoses communicate directly with the aneurysmal sac. Typical sources include the inferior and lumbar mesenteric arteries(22). This is the most common type of endoleak found after endovascular repair of abdominal aortic aneurysms. The number of patent collateral vessels and the amount of thrombi present in the aneurysmal sac observed in the preoperative period seems to be correlated with the risk for development of type II endoleaks. In cases of increased aneurysmal sac or false lumen patency persistence, percutaneous selective embolization is suggested, and is easily performed(22). Type III endoleak is secondary to the disconnection of the endoprosthesis elements, and requires immediate treatment in order to avoid severe complications caused by the continuous flow within the aneurysm or the false lumen. In such cases, endovascular therapy may be performed by means of the insertion of a new endoprosthesis inside the previous one. In more complex cases, surgical removal is the most appropriate approach(22). Repeated stress from arterial pulses on the prosthesis graft may cause such types of leaks. Additionally, as the aneurismal sac decreases along time, additional forces are applied on the graft, and may lead to failure. In spite of being very uncommon, type III endoleaks will probably become most prevalent in the long term follow-up of patients with endoprostheses(22). Type IV endoleaks are caused by the endoprostheses porosity. Such endoleaks are identified, at the moment of the prosthesis implantation, as a blurring observed at the immediate post-implantation angiogram, while the patients are under anti-coagulant drugs effect. Such endoleaks do not require specific intervention besides the wait for the restoration of normal coagulation times. The diagnosis of a type IV endoleak is based on exclusion, as other types of endoleaks may simulate it in the immediate post-implantation evaluation(22). Multidetector CT angiography is the imaging method of choice for the followup of these patients, because of the high sensitivity of the method in the identification of endoleaks and other complications associated with the procedure(19,25). Several authors have demonstrated that CT angiography is the best noninvasive method for the diagnosis of endoleaks, and it is considered as the gold standard and method of choice in such cases(26). With a single breath-hold following the contrast medium injection, the thoracic and abdominal aortas, supra-aortic vessels, abdominal branches and the iliaco-femoral axis can be evaluated. The images can be studied in the standard axial plane and can be later be processed through multiplanar reconstructions, utilizing different algorithms (VRT, MIP, MPR, shaded surface display [SSD], etc.) providing three-dimensional characterizations. CT angiography can provide the following data: aorta diameter and morphology, diameter and length of the distal and proximal necks, presence of thrombi or calcifications, abdominal branches patency, size, tortuosity and status of the iliac and femoral arteries disease. At ECG-gated CT angiography, volumetric three-dimensional data allow the rotation of the aorta, while visualizing it in different phases of the cardiac cycle. This resource may improve the diagnostic accuracy, as motion artifacts are, many times, the cause of false-positive results(24,27). Because of variable flow rates, endoleaks may not be detected at several moments following contrast injection. For this reason, multiphase CT angiography has been recommended: a typical protocol includes images before the administration of contrast medium, images after the administration of contrast in the arterial phase and images in the delayed phase. Pre-contrast images can be useful in the differentiation of calcifications in the endoleak aneurysmal sac, thus reducing the number of inconclusive studies(20,28). Recently, with the development of tomography apparatuses with 320 detector rows, new techniques for images acquisition have been developed. The literature has already reported the successful utilization of dynamic volumetric 4D CT angiography (DV-CTA) in selected cases, for a better characterization of the endoleaks and their appropriate treatment(29). However, other methods that do not utilize ionizing radiation have been gaining ground in the follow-up of such patients. Color Doppler US is well accepted in the follow-up of abdominal aortic aneurysms but, in spite of being noninvasive, widely available, inexpensive, easy to perform and non-ionizing method, its accuracy and reliability in the evaluation of aneurysms after endovascular repair is not well defined. Additionally, initial studies have demonstrated a limited success of this method(20,30). The development of sonographic contrast agents in association with softwares and specific contrast-enhanced imaging techniques (CEUS), have aroused new interest in this imaging method and its potential use for the routine follow-up of these patients. Most recently, studies in the literature have demonstrated that sonographic contrast agents seem to increase the sensitivity of the method in the post-endovascular repair follow-up, by increasing the blood flow echogenicity, allowing a better evaluation of possible complications of the graft, such as the case of endoleaks. On the other hand, the use of contrast-enhanced US does not replace CT angiography with regards to data related to the integrity of the frat anchoring, changes in the morphology of the aneurysmal sac and visceral vessels patency (30). Gadolinium-enhanced magnetic resonance imaging is capable of demonstrating endoleaks, but its performance depends upon the stent composition. Nitinol stents are generally more appropriate for magnetic resonance imaging; elgiloy stents may obscure the lumen and stainless steel stents cause extensive artifacts, which leads the study to be inconclusive. It should be highlighted that in several studies involving a small number of patients with a predominance of nitinol stents, MRI angiography was at least as sensitive as CT angiography, and in some cases, it demonstrated endoleaks that were not detected at CT angiography( 24). The ideal frequency for post-procedural imaging has not been systematically determined. Initial suggestions, based on empirical observations of abdominal aortic aneurysms, indicate the follow-up with CT angiography and radiography at one month and at six months after the repair and at every six months thereafter along the patient’s lifetime(23). CONCLUSION Early diagnosis and correct classification of endoleaks are crucial for an appropriate management of cases. The knowledge of endoleaks subtypes is fundamental in the education of physicians specialized in radiology and imaging diagnosis as well as for vascular surgeons. REFERENCES 1. Johnston KW, Rutherford RB, Tilson MD, et al. Suggested standards for reporting on arterial aneurysms. J Vasc Surg. 1991;13:452–8. 2. Brunkwall J, Hauksson H, Bengtsson H, et al. Solitary aneurysms of the iliac arterial system: an estimate of their frequency of occurrence. J Vasc Surg. 1989;10:381–4. 3. Bickerstaff LK, Hollier LH, Van Peenen HJ, et al. Abdominal aortic aneurysms: the changing natural history. J Vasc Surg. 1984;1:6–12. 4. Leopold GR, Goldberger LE, Bernstein EF. Ultrasonic detection and evaluation of abdominal aortic aneurysms. Surgery. 1972;72:939–45. 5. Law M. Screening for abdominal aortic aneurysms. Br Med Bull. 1998;54:903–13. 6. Thurmond AS, Semler HJ. Abdominal aortic aneurysm: incidence in a population at risk. J Cardiovasc Surg (Torino). 1986;27:457–60. 7. Treiman RL, Levine KA, Cohen JL, et al. Aneurysmectomy in the octogenarian. A study of morbidity and quality of survival. Am J Surg. 1982; 144:194–7. 8. Albuquerque ELC, Braile DM, Palma JH, et al. Diretrizes para o tratamento cirúrgico das doenças da aorta da Sociedade Brasileira de Cirurgia Cardiovascular. Rev Bras Cir Cardiovasc. 2007;22: 137–59. 9. Parodi JC, Palmaz JC, Barone HD. Transfemoral intraluminal graft implantation for abdominal aortic aneurysms. Ann Vasc Surg. 1991;5:491–9. 10. EVAR trial participants. Endovascular aneurysm repair versus open repair in patients with abdominal aortic aneurysm (EVAR trial 1): randomised controlled trial. Lancet. 2005;365:2179–86. 11. Greenberg RK, Clair D, Srivastava S, et al. Should patients with challenging anatomy be offered endovascular aneurysm repair? J Vasc Surg. 2003; 38:990–6. 12. Hallett JW Jr. Management of abdominal aortic aneurysms. Mayo Clin Proc. 2000;75:395–9. 13. Sampaio SM, Shin SH, Panneton JM, et al. Intraoperative endoleak during EVAR: frequency, nature, and significance. Vasc Endovascular Surg. 2009;43:352–9. 14. Lumsden AB, Allen RC, Chaikof EL, et al. Delayed rupture of aortic aneurysms following endovascular stent grafting. Am J Surg. 1995;170: 174–8. 15. Alimi YS, Chakfe N, Rivoal E, et al. Rupture of an abdominal aortic aneurysm after endovascular graft placement and aneurysm size reduction. J Vasc Surg. 1998;28:178–83. 16. Torsello GB, Klenk E, Kasprzak B, et al. Rupture of abdominal aortic aneurysm previously treated by endovascular stentgraft. J Vasc Surg. 1998;28: 184–7. 17. Harris PL, Vallabhaneni SR, Desgranges P, et al. Incidence and risk factors of late rupture, conversion, and death after endovascular repair of infrarenal aortic aneurysms: the EUROSTAR experi ence. European Collaborators on Stent/graft techniques for aortic aneurysm repair. J Vasc Surg. 2000;32:739–49. 18. Zarins CK, Wolf YG, Lee WA, et al. Will endovascular repair replace open surgery for abdominal aortic aneurysm repair? Ann Surg. 2000;232: 501–7. 19. White GH, Yu W, May J, et al. Endoleak as a complication of endoluminal grafting of abdominal aortic aneurysms: classification, incidence, diagnosis, and management. J Endovasc Surg. 1997; 4:152–68. 20. Golzarian J, Dussaussois L, Abada HT, et al. Helical CT of aorta after endoluminal stent-graft therapy: value of biphasic acquisition. AJR Am J Roentgenol. 1998;171:329–31. 21. Iezzi R, Cotroneo AR, Basilico R, et al. Endoleaks after endovascular repair of abdominal aortic aneurysm: value of CEUS. Abdom Imaging. 2010; 35:106–14. 22. Fanelli F, Dake MD. Standard of practice for the endovascular treatment of thoracic aortic aneurysms and type B dissections. Cardiovasc Intervent Radiol. 2009;32:849–60. 23. Ueda T, Fleischmann D, Dake MD, et al. Incomplete endograft apposition to the aortic arch: birdbeak configuration increases risk of endoleak formation after thoracic endovascular aortic repair. Radiology. 2010;255:645–52. 24. Pannu HK, Jacobs JE, Lai S, et al. Gated cardiac imaging of the aortic valve on 64-slice multidetector row computed tomography: preliminary observations. J Comput Assist Tomogr. 2006;30: 443–446. 25. White GH, Yu W, May J. Endoleak – a proposed new terminology to describe incomplete aneurysm exclusion by an endoluminal graft. J Endovasc Surg. 1996;3:124–5. 26. Thomaz FB, Lopez GE, Marchiori E, et al. Avaliação pós-operatória do tratamento endovascular de aneurisma da aorta abdominal por angiotomografia com mu tidetectores. Radiol Bras. 2008;41:213–7. 27. Zhang J, Fletcher JG, Vrtiska TJ, et al. Large- vessel distensibility measurement with electro cardiographically gated multidetector CT phantom study and initial experience. Radiology. 207; 245:258–66. 28. Bley TA, Chase PJ, Reeder SB, et al. Endovascular abdominal aortic aneurysm repair: nonenhanced volumetric CT for follow-up. Radiology. 2009;253:253–62. 29. Bent CL, Jaskolka JD, Lindsa y TF, et al. The use of dynamic volumetric CT angiography (DV-CTA) for the characterization of endoleaks following fenestrated endovascular aortic aneurysm repair (f-EVAR). J Vasc Surg. 2010;51:203–6. 30. Mirza TA, Karthikesalingam A, Jackson D, et al. Duplex ultrasound and contrast-enhanced ultrasound versus computed tomography for the detection of endoleak after EVAR: systematic review and bivariate meta-analysis. Eur J Vasc Endovasc Surg. 2010;39:418–28. 1. MDs, Residents, Division of Radiology, Department of Medical Practice – Faculdade de Medicina de Ribeirão Preto da Universidade de São Paulo (FMRP-USP), Ribeirão Preto, SP, Brazil. 2. Graduate Student at Faculdade de Medicina da Universidade Federal do Ceará (UFC), Sobral, CE, Brazil. 3. MD, Resident of Neurology at Hospital Geral de Fortaleza (HGF), Fortaleza, CE, Brazil. 4. Professor Doctor, Division of Radiology, Department of Medical Practice at Faculdade de Medicina de Ribeirão Preto da Universidade de São Paulo (FMRP-USP), Ribeirão Preto, SP, Brazil. 5. Professor Doctor, Division of Radiology, Coordinator of the Center of Imaging Sciences and Medical Physics at Hospital das Clínicas da Faculdade de Medicina de Ribeirão Preto da Universidade de São Paulo (HCFMRP-USP), Ribeirão Preto, SP, Brazil. Mailing address: Dr. Francisco Abaeté das Chagas Neto Secretaria do Setor de Radiologia (CCIFM), Hospital das Clínicas – FMRPUSP Avenida Bandeirantes, 3900, Campus Universitário, Monte Alegre Ribeirão Preto, SP, Brazil, 14048-900 E-mail: abaeteneto@yahoo.com.br Received February 17, 2010 Accepted after revision August 6, 2010 Study developed in the Center of Imaging Sciences and Medical Physics at Hospital das Clínicas da Faculdade de Medicina de Ribeirão Preto da Universidade de São Paulo (HCFMRPUSP), Ribeirão Preto, SP, Brazil. Financial support: Fundação de Apoio ao Ensino, Pesquisa e Assistência do Hospital das Clínicas da Faculdade de Medicina de Ribeirão Preto da Universidade de São Paulo (FAEPA) |
|
Av. Paulista, 37 - 7° andar - Conj. 71 - CEP 01311-902 - São Paulo - SP - Brazil - Phone: (11) 3372-4544 - Fax: (11) 3372-4554