Radiologia Brasileira - Publicação Científica Oficial do Colégio Brasileiro de Radiologia
AMB - Associação Médica Brasileira CNA - Comissão Nacional de Acreditação
 Vol. 46 nº 1 - Jan. /Feb. of 2013
Vol. 46 nº 1 - Jan. /Feb. of 2013
|
ORIGINAL ARTICLE
|
|
Carpal tunnel syndrome: comparative study between sonographic and surgical measurements of the median nerve in moderate and severe cases of disease |
|
|
Autho(rs): Marcelo de Pinho Teixeira Alves1; Clovis Orlando Pereira da Fonseca2; José Mauro Granjeiro3; Paulo Roberto Gonçalves de Souza4; Marcos Tzirulnik5 |
|
|
Keywords: Carpal tunnel syndrome; Ultrasonography; Diagnosis; Neuropathy. |
|
|
Abstract: INTRODUCTION
In most cases, carpal tunnel syndrome (CTS) can be diagnosed only by clinical history and physical examination(1,2). In spite of being accepted as the gold standard for diagnosis, electromyography (EMG) has a false-negative rate of 10% to 20%(1-4) and does not provide information on the median nerve (MN) itself and surrounding tissues, which may be relevant in the determination of the etiologic diagnosis. Over the last years, magnetic resonance imaging and ultrasonography became noninvasive imaging methods(5) capable of demonstrating many characteristics of soft tissues, such as change in the MN signal, increase of its cross sectional area, nerve flattening, and bowing of the flexor retinaculum(4). In the case of ultrasonography, it is important to highlight its low cost and objectivity. Many authors have affirmed that the sonographic measurement of the median nerve at the level of the proximal edge of the carpal tunnel, next to the pisiform bone, is a good alternative to EMG in the diagnosis of CTS, eventually becoming a substitute for the latter(6-18). So far, there are no references in the literature about comparative studies between sonographic measurement and actual surgical measurement of the MN in cases of CTS, observed during carpal tunnel release. This study is aimed at: 1) comparing the MN sonographic and surgical perimeters; 2) evaluating the CTS diagnosis by means of the median nerve cross-sectional area (MNSA); and 3) evaluating the association between MNSA and CTS severity. MATERIALS AND METHODS The present study was developed according to the Resolution CNS 196/96 and the Helsinki Declaration (Somerset-West Amendment, South Africa) and was approved by the Committee for Ethics in Research of the institution (Resolution CAAE 0208.0.258.000-09, June 11, 2009). Between May, 2010 and June, 2011, the authors evaluated 30 patients diagnosed with CTS. All the patients signed a term of free and informed consent. The inclusion criteria were the following: CTS diagnosed by EMG and clinically; minimum of 12 months with the disease; failure of at least 6 months of conservative treatment; minimum age of 18 years at the moment of the diagnosis. Patients with previous trauma in the wrist were excluded, as well as pregnant and lactating women, patients with previous surgical treatment or corticoid injection in the wrist, infection, systemic disease without appropriate management which might interfere with the study, myocardial infarction less than 6 months before the study, and anatomic variation of the MN seen at ultrasonography. Besides EMG findings, clinical history (presence of paresthesia, nocturnal pain) and physical examination findings (Tinel's, Phalen's and Durkan's tests, and presence of thenar atrophy) were evaluated. The EMG was considered as positive whenever the sensory latency was > 3.2 milliseconds (ms) and/or motor latency > 4.0 ms. The electromyography was performed in compliance with the standards of the Neurology Department of the institution. All the patients were clinically evaluated by a single observer and classified according to Gelberman et al. for CTS severity(19), as follows: stage zero - healthy patient with no symptom; stage 1 (early CTS) - symptoms lasting less than a year without any permanent sensory deficit; stage 2 (intermediate CTS) - paresthesia or numbness, permanent or intermittent pain, nocturnal pain and/or pain exacerbated by daily activities; stage 3 (advanced CTS) - same as stage 2, associated with thenar atrophy(19,20). Only those patients at stages 2 and 3, with positive EMG, were included. Wrist ultrasonography was performed always in the most symptomatic wrist of the patient, at ambient temperature. The technique was standardized and utilized in all the patients (patient in a sitting position, forearm supine resting on a table, neutral wrist position and fingers in resting, semiflexed position)(12,13,20,21). The nerve measurement was performed with the sonographic transducer placed on the wrist, at one centimeter distal from the proximal flexion fold. The pisiform bone knobs and the scaphoid tubercle are identified by palpation, without any compressive force. A Toshiba apparatus with a multifrequency 12 MHz transducer (Figure 1) and a General Electric apparatus with a 11 MHz transducer were utilized to acquire cross sectional wrist images for evaluating the MN cross sectional area at the entrance of the carpal tunnel, considering the pisiform bone as a fixed parameter. The sonographic perimeter of the MN was obtained by drawing a continuous line around the boundary of the nerve(12,13); the cross-sectional area of the MN SA was automatically calculated by the ultrasonography equipment. The nerve margin was defined as the external margin of the hypoechoic nerve fascicles and the interior of the hyperechoic nerve sheath(13,22). The radiologist was blind to the patients' clinical data and EMG results. 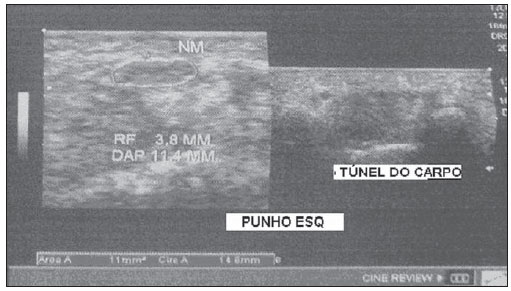 Figure 1. Ultrasonography with Toshiba equipment. The cross-sectional area was selected as the standard measurement of the MN for the purposes of CTS diagnosis, and was measured in square centimeters (cm2), and 0.09 cm2 as cutoff point for CTS diagnosis in patients at intermediate or advanced stages of CTS. The following sonographic classification developed by El Miedany et al.(12,21)was adopted as a standard for the present study: early CTS - SA = 0.09 cm2 and < 0.10 cm2; mild CTS - SA = 0.10 cm2 and < 0.13 cm2; moderate CTS - SA = 0.13 cm2 and < 0.15 cm2; severe CTS - SA = 0.15 cm2. A single surgeon operated all the patients, in order to standardize the surgical technique. The anterior longitudinal access previously described by Ortiz et al.(23,24)was utilized after exsanguination of the limb, placement of the pneumatic cuff and brachial plexus block or regional intravenous anesthesia. The external neurolysis of the MN was performed, allowing for the measurement of the MN surgical perimeter (Figure 2). Such measurement was performed by means of a suture thread placed around the nerve in the same location of the sonographic measurement. In order to avoid measurement errors, such procedure was repeated three times, with different suture threads, and the mean value of the three measurements was adopted. The suture line was placed along a simple millimeter ruler, thus obtaining the nerve perimeter in millimeters. The same ruler was utilized for all the measurements, and its percentage error was verified by Inmetro. 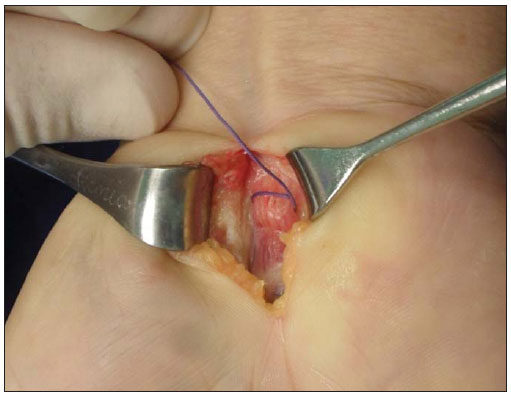 Figure 2. Measurement of the surgical perimeter of the median nerve. After the nerve measurement, the wound was cleaned, and the incision was closed. No complication was observed. Low-intensity laser therapy was utilized during the postoperative recovery period(25). The correlations between the classifications according to Gelberman et al. (clinical) and El Miedany et al. (sonographic) were analyzed for the purposes of clinical/sonographic correlation and evaluation of CTS severity. The sonographic and surgical measurements of the MN were compared. The clinical classification was compared with the sonographic perimeter, cross-sectional area and surgical perimeter of the MN. The statistical analyses included paired samples t-test, Pearson's correlation, Bland Altman's diagram to study the correlation between perimeters, Komolgorov-Smirnov's test for composite normality, Welch and Wilcoxon's tests to compare clinical severity and measurements. RESULTS Out of the 30 initial patients, five cases were lost due to lack of recordings as follows: MN sonographic perimeter (one case), anatomic variations of the MN seen at ultrasonography (bifid MN, three cases), and latency values at EMG (one case). The mean age of the patients include in the present study was 54 years, and the mean course of CTS was 25 months, with a predominance of female patients (92%). Durkan's test was positive in 92% of the patients, followed by Tinel's test, positive in 88% of the patients. Phalen's test was positive in 76% of the patients. Paresthesia was the predominant symptom, observed in 88% of the patients. Only 68% of the patients complained of nocturnal pain, and the occurrence of thenar atrophy was observed in 40% of the patients. The sonographic SA, as well as the sonographic and surgical perimeters are shown on Figures 3 and 4, respectively. The MN cross-sectional area SA was 0.09 cm2 in only one patient. In the present study, all the other patients presented SA > 0.09 cm2.  Figure 3. Sonographic and surgical perimeters of the median nerve (p < 0.001). 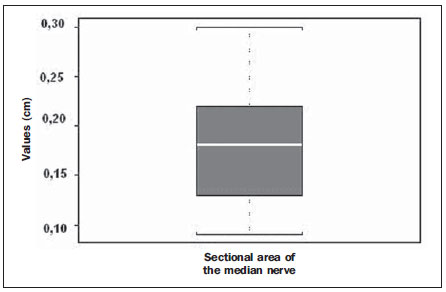 Figure 4. Sonographic sectional area of the median nerve of patients in the present study. As one may observe in the clinical classification proposed by Gelberman et al., there was a predominance of patients at stage 2 (60% of the patients) (Table 1). 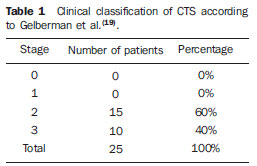 The El Miedany sonographic classification for CTS severity is shown on Table 2. One observes a predominance of patients with severe CTS (SA > 0.15 cm2) (15 cases, 60%), only 1 patient (4%) with SA = 0.09 cm2, 4 patients (16%) with mild CTS, and 5 patients (20%) with moderate CTS. 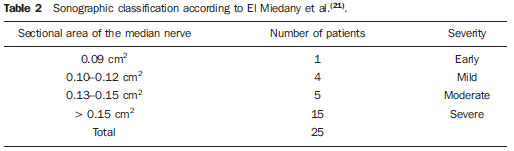 In a comparison between the classifications proposed by Gelberman et al. and El Miedany et al., as shown on Table 3, among the 15 patients at stage 2 (moderate CTS), 7 presented severe CTS at ultrasonography (SA > 0.15 cm2; 47% of the patients), 1 patient (7%) presented incipient CTS, 4 patients (27%) presented mild CTS and 3 patients (20%) presented moderate CTS. Among the 10 patients at Gelberman et al. stage 3, 8 (80%) presented severe CTS at ultrasonography, while 2 patients presented moderate CTS. 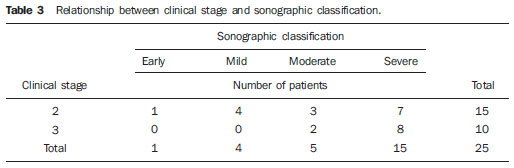 On Table 4, which compares the Gelberman et al. classification with the mean value of the MN measurements, one observes that, at stage 3, the mean MN measurements were greater than the mean measurements at stage 2. The mean value of MN's surgical perimeter at stage 2 was 21.8 (± 2.78) mm, as compared with 22.7 (± 3.16) mm at stage 3. The cross-sectional area at 3, 0.20 ± 0.06 cm2. The sonographic perimeter was 18.25 ± 3.15 mm at stage 2 and 19.75 ± 2.46 mm at stage 3. 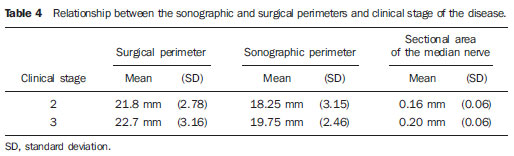 In the present study sample, the Kolmogorov-Smirnov test for composite normality did not present a normal distribution for surgical perimeters (p = 0), but normal distribution was observed for sonographic perimeters (p = 0.5). The paired t-test did not identify any agreement between surgical and sonographic perimeters, with significant differences between both (confidence interval of 95%; p < 0.001). Such difference between the perimeters can be observed on Figure 4. Pearson's correlation coefficient between surgical and sonographic perimeters was 0.3913. As shown on Figure 5 (Bland-Altman diagram), the surgical perimeters were consistently higher than the sonographic perimeters, suggesting some proportionality between both of them. However, with basis on the simple linear regression model, according to the surgical perimeter regression coefficient (regression coefficient 0.18; p = 0.3913), one observed that there was no statistically significant association between the perimeters, and that there was no proportionality between them.  Figure 5. Bland-Altman diagram. The Welch's modified two-sample test (p = 0.0983; confidence interval [CI] of 95%) (Figure 6) did not demonstrate statistically significant association between the two clinical stages and the cross-sectional areas at ultrasonography, probably because of the low number of patients included in the study sample. The mean cross-sectional area at clinical stage 3 is higher than at clinical stage 2. 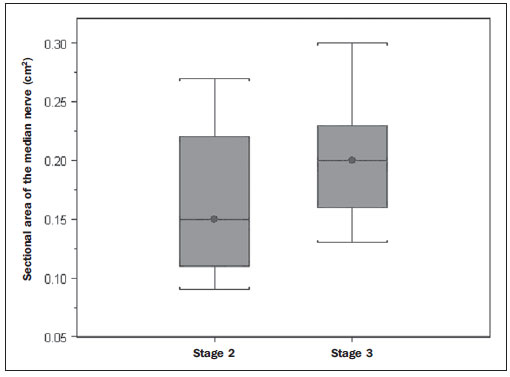 Figure 6. Sectional areas of the median nerve related to clinical stages of the disease. There was neither statistically significant association between sonographic perimeter and the clinical stage of CTS (Welch's modified two-sample test, p = 0.1956; CI of 95%) (Figure 7), nor between the surgical perimeter and clinical stage of CTS (Wilcoxon's test, p = 0.1487; CI of 95%) (Figure 8).  Figure 7. Sonographic perimeter of the median nerve and clinical stages of the disease.  Figure 8. Surgical perimeters of the median nerve related to clinical stage of the disease. DISCUSSION Carpal tunnel syndrome is the most common peripheral compression neuropathy, occurring in 0.1% to 10% of the population, and in most of cases is accurately diagnosed in the presence of association of pain, nocturnal numbness and positive Tinel's, Phalen's and Durkan's tests(1), the latter being most sensitive for detecting CTS at physical examination(3). In the present study, there was predominance of female patients (92%) and patients above the age of 40 years (mean age of 54 years), in agreement with the literature(1-3,6). Paresthesia was the most common symptom (88% of the cases). As the most common results, positive Durkan's test (92% of the cases) and Tinel's sign (88% of the cases) were also in agreement with the literature(1-3). As observed, Durkan's test was the most sensitive for CTS diagnosis. The authors found 40% of the patients with thenar eminence atrophy, contributing to a higher severity of the disease. Such a fact can be a consequence of a selection bias, as all the patients were previously diagnosed with CTS and had been referred for surgical evaluation in a specialized service. Currently, (EMG is the most appropriate complementary method for CTS diagnosis, in spite of its false-negative rate. Some authors advocate that ultrasonography may substitute or complement EMG in the CTS diagnosis(7-18,20). In such case, the use of a sonographic transducer of at least 10 MHz(26) is recommended, because of higher image definition and clearer outlining of the MN. The measurement of the MN's cross-sectional area is better performed by acquiring the perimeter of the nerve(12,13,21). A cross-sectional area > 0.09 cm2 at the entrance of the carpal tunnel is considered as being valid for the diagnosis of CTS(6,13,26). Indeed, the standardization in the performance of the ultrasonography studies was an important factor to avoid possible errors in the measurement of the MN's measures, as highlighted in the study developed by Carvalho et al.(27), allowing the utilization of different ultrasonography apparatuses for the studies. In the present study, two different transducers with excellent image definition were utilized, adopting 0.09 cm2 as the cutoff point in the case of patients at intermediate or advanced stages of CTS; in only one patient was such value found (4% of the cases) with all the remaining ones (96%) presenting higher values for MN cross-sectional area. The study developed by Carvalho et al.(27) reviewing published studies related to sonographic diagnosis of CTS has reported that the most frequently observed cutoff point was between 0.09 cm2 and 0.10 cm2 (in those studies the measurements were taken in mm2 and, in the present study, were converted into cm2). Thus, the authors conclude that a value corresponding to 0.09 cm2 for MN cross-sectional area is valid for the diagnosis of CTS in patients with clinical suspicion of intermediate (moderate) or advanced (severe) stages of disease, avoiding EMG for such patients. In the study published by Klauser et al.(28) a second measurement of the median nerve cross-sectional area was included, adopting the difference between the cross-sectional areas measured in the carpal tunnel and proximal to the pronator quadratus as the best diagnostic parameter, utilizing the same technique described in the present study for measurement of the cross-sectional area in the carpal tunnel. Those authors have concluded that such a difference would be the best diagnostic parameter, reducing the possibility of false-positive results. In the present study, only patients at intermediate (moderate) and advanced (severe) stages of disease were included. For a future perspective including patients at earlier stages of the disease, it is suggested that the evaluation of the difference between measurements may improve the capability of ultrasonography for the diagnosis of CTS. The importance of the clinical classification proposed by Gelberman et al. lies on the fact that it allows estimating which patients are to be referred either to surgical or nonsurgical treatment. Patients with less than 12 months from symptoms onset, with no definitive sensory deficit, may benefit from nonsurgical treatment, generally with the utilization of steroid or nonsteroidal anti-inflammatory drugs in association with physiotherapy or corticoid infiltration in the carpal tunnel. Such treatment may succeed in up to 40% of the cases(1,3). In the present study, such cases were excluded, and 60% of the patients were at stage 2, and 40% were at stage 3 of the disease. According to some authors(12,21), ultrasonography can identify the most severe cases of CTS related to increase in the MN's cross-sectional area. In the present study, the authors found 20 cases of greater severity at ultrasonography [moderate 5 (20%); severe 15 (60%)]. The present study has compared such two classifications. In spite of the high number of severe cases identified at ultrasonography, no correlation was observed between clinical stages and sonographic cross-sectional area of MNs, as well as sonographic and surgical perimeters. The p value was not significant for the purpose of comparison between MN's cross-sectional area, surgical perimeter, sonographic perimeter and clinical stages of CTS. Thus, it was not possible to confirm the association between sonographic severity and clinical severity of CTS in the present study sample. The simple linear regression ruled out the proportionality between the NM's measurements, in spite of the intuitive thought of larger measurements after surgical nerve release. This is the first study approaching the correlation between the sonographic and surgical perimeters in CTS. The surgical perimeter was considered as an actual measurement of the MN, since the nerve was measured during a surgery and then confirmed with a certified ruler. The percentage error of such ruler was 0.3%, therefore a significant degree of accuracy. In the present study, the authors observed that, generally, the sonographic perimeter is smaller than the surgical perimeter, with no association between both perimeters and clinical stages of CTS. Considering the innovative nature of the present study, being the first one comparing sonographic and surgical measurements of the median nerve, further studies would be required to assess the NM measurements in other diseases different from CTS, with the purpose of verifying whether changes in the MN perimeter occur after carpal tunnel release in patients without compressive disorders of the nerve in the wrist. The cost of the treatment for CTS, both in public and private institutions, is a relevant socio-economic factor to be taken into consideration(13,17,20). In the present study, the authors observed that the diagnosis of CTS by means of ultrasonography in patients with clinical suspicion of moderate or severe (advanced) disease is a way to reduce treatment costs. A MN cross-sectional area = 0.09 cm2 is a parameter for diagnosis of CTS, according to the data presented in this study, with EMG remaining reserved for patients with suspicion of other diseases which may confuse the investigator, or for those patients at early clinical stages of the disease. However, according to Carvalho et al.(27), CTS should be ruled out in cases where the MN cross-sectional area is < 0.07 cm2 or 0.08 cm2; on the other hand, CTS should be confirmed in cases where the cross-sectional area is > 0.13 cm2 or 0.14 cm2, even in those patients at earlier clinical stages of CTS. Based on the results of the present study, one can infer the impact caused by studies with larger samples. In public health services where EMG is not widely available, and in those cases where the patients must be transported to larger cities where EMG is available, the utilization of ultrasonography can contribute to a significant reduction of the social costs of CTS. CONCLUSIONS 1. No association was observed between sonographic and surgical perimeters of the median nerve in the present study; 2. The sonographic measurement of the MN's cross-sectional area is valid for the diagnosis of CTS in patients at moderate (intermediate) and severe stages of the disease; 3. In the present study sample, no association was observed between clinical stage of CTS and cross-sectional area of the median nerve. REFERENCES 1. Parisi DM, Trumble TE. Wrist and hand reconstruction. In: AAOS Orthopaedic Knowledge Update 8;2005. p. 351. 2. Trumble TE, Diao E, Abrams RA, et al. Single portal endoscopic carpal tunnel release compared with open release: a prospective, randomized trial. J Bone Joint Surg Am. 2002;84-A:1107-15. 3. Howard RF. Hand and microsurgery. In: Miller MD, editor. Review of orthopaedics. Philadelphia, PA: Saunders; 2004. p. 358. 4. Mallouhi A, Pülzl P, Trieb T, et al. Predictors of carpal tunnel syndrome: accuracy of gray-scale and color Doppler sonography. AJR Am J Roentgenol. 2006;186:1240-5. 5. Alves MPT, Moraes Neto GP, Tzirulnik M. Avaliação clínico-ultra-sonográfica da tenossinovite estenosante de De Quervain. Rev Bras Ortop. 2000;35:118-22. 6. Moran L, Perez M, Esteban A. et al. Sonographic measurement of cross-sectional area of the median nerve in the diagnosis of carpal tunnel syndrome. correlation with nerve conduction studies. J Clin Ultrasound. 2009;37:125-31. 7. Wang LY, Leong CP, Huang YC, et al. Best diagnostic criterion in high-resolution ultrasonography for carpal tunnel syndrome. Chang Gung Med J. 2008;31:469-76. 8. Ashraf AR, Jali R, Moghtaderi AR. et al. The diagnostic value of ultrasonography in patients with electrophysiologically confirmed carpal tunnel syndrome. Electromyogr Clin Neurophysiol. 2009;49:3-8. 9. Pastare D, Therimadasamy AK, Lee E, et al. Sonography versus nerve conduction studies in patients referred with a clinical diagnosis of carpal tunnel syndrome. J Clin Ultrasound. 2009;37:389-93. 10. Hobson-Webb LD, Padua L. Median nerve ultrasonography in carpal tunnel syndrome: findings from two laboratories. Muscle Nerve. 2009;40:94-7. 11. Karadağ YS, Karadağ O, Ciçekli E, et al. Severity of carpal tunnel syndrome assessed with high frequency ultrasonography. Rheumatol Int. 2010;30:761-5. 12. Mondelli M, Filippou G, Gallo A, et al. Diagnostic utility of ultrasonography versus nerve conduction studies in mild carpal tunnel syndrome. Arthritis Rheum. 2008;59:357-66. 13. Ziswiler HR, Reichenbach S, VÖgelin E, et al. Diagnostic value of sonography in patients with suspected carpal tunnel syndrome: a prospective study. Arthritis Rheum. 2005;52:304-11. 14. Duncan I, Sullivan P, Lomas F. Sonography in the diagnosis of carpal tunnel syndrome. AJR Am J Roentgenol. 1999;173:681-4. 15. Buchberger W, Judmaier W, Birbamer G, et al. Carpal tunnel syndrome: diagnosis with high-resolution sonography. AJR Am J Roentgenol. 1992;159:793-8. 16. Wong SM, Griffith JF, Hui ACF, et al. Carpal tunnel syndrome: diagnostic usefulness of sonography. Radiology. 2004;232:93-9. 17. Wong SM, Griffith JF, Hui ACF, et al. Discriminatory sonographic criteria for the diagnosis of carpal tunnel syndrome. Arthritis Rheum. 2002;46:1914-21. 18. Chen P, Maklad N, Redwine M, et al. Dynamic high-resolution sonography of the carpal tunnel. AJR Am J Roentgenol. 1997;168:533-7. 19. Gelberman RH, Rydevik BL, Pess GM, et al. Carpal tunnel syndrome. A scientific basis for clinical care. Orthop Clin North Am. 1988;19:115-24. 20. Colak A, Kutlay M, Pekkafali Z, et al. Use of sonography in carpal tunnel surgery. A prospective study. Neurol Med Chir (Tokyo). 2007;47:109-15. 21. El Miedany YM, Aty SA, Ashour S. Ultrasonography versus nerve conduction study in patients with carpal tunnel syndrome: substantive or complementary tests? Rheumatology (Oxford). 2004;43:887-95. 22. Kwon BC, Jung KI, Baek GH. Comparison of sonography and electrodiagnostic testing in the diagnosis of carpal tunnel syndrome. J Hand Surg Am. 2008;33:65-71. 23. Ortiz J, Llobet AJ. Síndrome do canal carpiano: tratamento cirúrgico por miniincisão. Rev Bras Ortop. 1990;25:50-4. 24. Alves MPT. Liberação do canal do carpo por mini-incisão transversa. Acta Ortop Bras. 2011;19:362-7. 25. Alves MPT, Araújo GCS. Laserterapia de baixa intensidade no pós-operatório da síndrome do túnel do carpo. Rev Bras Ortop. 2011;46:697-701. 26. Kotevoglu N, Gülbahce-Saglam S. Ultrasound imaging in the diagnosis of carpal tunnel syndrome and its relevance to clinical evaluation. Joint Bone Spine. 2005;72:142-5. 27. Carvalho KMD, Soriano EP, Carvalho MVD, et al. Nível de evidência e grau de recomendação dos artigos sobre a acurácia diagnóstica da ultrassonografia na síndrome do túnel do carpo. Radiol Bras. 2011;44:85-9. 28. Klauser AS, Halpern EJ, De Zordo T, et al. Carpal tunnel syndrome assessment with US: value of additional cross-sectional area measurements of the median nerve in patients versus healthy volunteers. Radiology. 2009;250:171-7. 1. MD, MsC, Orthopedist and Hand Surgeon, Universidade Federal Fluminense (UFF), Niterói, RJ, Brazil. 2. PhD, Professor of Neurosurgery, Universidade Federal Fluminense (UFF), Niterói, RJ, Brazil. 3. PhD, Senior Researcher, Professor, Universidade Federal Fluminense (UFF), Niterói, RJ, Brazil. 4. MD, Orthopedist and Hand Surgeon, Professor of Orthopedics, Universidade Federal Fluminense (UFF), Niterói, RJ, Brazil. 5. MD, MsC, Radiologist, Clínica de Imagem Marcos Tzirulnik – CIMT, Rio de Janeiro, RJ, Brasil. Mailing Address: Dr. Marcelo de Pinho Teixeira Alves Avenida Genaro de Carvalho, 2597, casa 1, Recreio dos Bandeirantes Rio de Janeiro, RJ, Brazil, 22795-077 E-mail: marceloptalves@hotmail.com Received August 22, 2012. Accepted after revision November 26, 2012. * Study developed at Hospital Universitário Antônio Pedro (HUAP) – Universidade Federal Fluminense (UFF), Niterói, RJ, Brazil. |
|
Av. Paulista, 37 - 7° andar - Conj. 71 - CEP 01311-902 - São Paulo - SP - Brazil - Phone: (11) 3372-4544 - Fax: (11) 3372-4554